The microporous membrane filtration method, as an important means of sterilization and particle removal, has been widely used in pharmaceuticals, particle detection, and sample processing. Syringe filter, as one of the most common laboratory consumables, in addition to undergo dissolution testing and chemical compatibility testing, integrity testing should also be performed when performing Quality Control on a Syringe Filter
The integrity of the laboratory syringe filter is a crucial indicator in filtration products. Whether a filtration system can maintain its integrity before and after filtration is an important parameter for assessing the quality of a filtration product. For filtration processes, especially sterilizing-grade filtration, integrity testing is a necessary means to ensure the safety and reliability of filtration, preventing unnecessary waste of time, effort, and filtration samples. Through integrity testing, the following can be ensured:
- The integrity of the laboratorysyringe filter and the tightness of the filtration system
- The filter membrane is intact and defect-free, and the selected membrane meets the filtration requirements.
- Correct installation and operation of thelaboratory syringe filter during the process
- The installed laboratorysyringe filter meets the standards provided by the manufacturer
There are two methods for integrity testing: destructive and non-destructive. Destructive testing refers to bacterial challenge testing, which is the fundamental method to prove that the laboratory syringe filter can meet the stringent standards of sterilizing-grade filters. In bacterial challenge testing, a certain number of samples are statistically selected from each batch of products and subjected to a bacterial challenge test using a Brevundimonas diminuta solution, following standard testing methods. Typically, the manufacturer of the filter materials conducts this type of destructive testing to check whether the product quality is acceptable.
Non-destructive testing methods mainly include bubble point testing (also known as pressure hold testing) and diffusion testing. For users of small filter products, the bubble point test is a simple and easy method. Through bubble point testing, the performance of the selected filter product can be evaluated, and the validity of the filtration results can be determined.
Bubble point testing is based on the capillary model. The structure of the filter membrane is filled with microporous channels, which are the basic units of filtration. These microporous channels act like many “capillaries.” When the filter membrane is fully wetted by a liquid, the liquid is retained within these “capillaries” due to surface tension. To expel the liquid from the membrane pores, an external gas pressure must be applied to overcome the surface tension. The minimum pressure required to completely expel the liquid from the membrane pores is the bubble point of the filter membrane, commonly referred to as the bubble point.The testing method based on this principle is known as the bubble point test. This is also the most widely used non-destructive integrity testing method. The bubble point value can be calculated using the following formula:
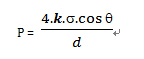
Where:
P = bubble point pressure𝑑
d = pore diameter𝑘
k = shape correction factor𝑞
q = liquid-solid contact angle𝑠
s = surface tension
The bubble point value is directly related to the pore size of the filter. There are many micropores on the filter membrane, and the bubble point value of each pore may not be exactly the same. The bubble point value of the membrane refers to the bubble point of the largest pore on the membrane, which is the bubble point of the pore with the largest diameter (where the liquid is most easily expelled to form a channel). When the bubble point is reached, at least one pore in the fully wetted membrane has its liquid completely expelled, forming a gas channel. Therefore, gas will quickly pass through this pore and flow to the downstream side of the membrane, forming continuous and stable bubbles. This sudden change in downstream gas flow indicates that the bubble point has been reached.For larger-area filters, the greater diffusion flow can make it difficult to determine the bubble point accurately. Therefore, for large-area filters, manual testing using diffusion flow testing is recommended. For small-area filters, the bubble point can be directly related to the pore size of the filter, making bubble point testing a quick and convenient method.
The bubble point value is a characteristic indicator of a filtration medium. For a filter membrane of a specific material and pore size, the bubble point value is theoretically a fixed value. Due to measurement errors and other factors, the measured value will fall within a fixed range. Typically, filter membrane manufacturers will specify the minimum bubble point value for a particular filter membrane.
Laboratory syringe filters are widely used by researchers in analytical and life sciences laboratories due to their simplicity and convenience. These seemingly simple small filters play a crucial role in experimental procedures. They ensure the smooth progress of subsequent experiments and the accuracy of experimental data. Additionally, in chromatographic analysis, they protect the lifespan of precision instruments and reduces the risk of damage by removing impurities in the loading sample, thereby maximizing cost savings.
Users may have some concerns when using laboratory syringe filters. Since the filter membrane is sealed inside and cannot be seen, how can one determine if a qualified membrane is used in the filter? Is the membrane properly sealed without any damage? Indeed, if an inferior quality laboratory syringe filter is used, the sample merely passes through the filter without the impurities being fully retained by the membrane. If the membrane is damaged or not completely sealed, the liquid can leak through gaps, causing subsequent experiments to fail and wasting resources.These concerns can be easily addressed by using the bubble point principle mentioned earlier to verify the quality of the laboratory syringe filter. Many reputable suppliers of laboratory syringe filter offer bubble point testers for small filters.
If the bubble point value measured by the bubble point tester is significantly lower than the specified value for the filter membrane, it indicates that the laboratory syringe filter may have significant issues, which could include:Membrane damage: The membrane may be damaged.Sealing defects: There may be gaps between the membrane and the filter due to sealing defects.Leakage in the filtration system: There may be leaks in the filtration system.Membrane material issues: There may be issues with the membrane material.Conversely, if the measured value equals or exceeds the bubble point specified by the supplier, it indicates that the filtration system is intact and effective, and the filtration effect is reliable.


Thanks for sharing! PES Syringe Filters offer reliable contamination-free filtration, especially in sterile applications. Their excellent chemical resistance and high throughput make them a top choice for researchers. Keep up the informative content!